Personalized medicine approaches designed with Fruit Flies
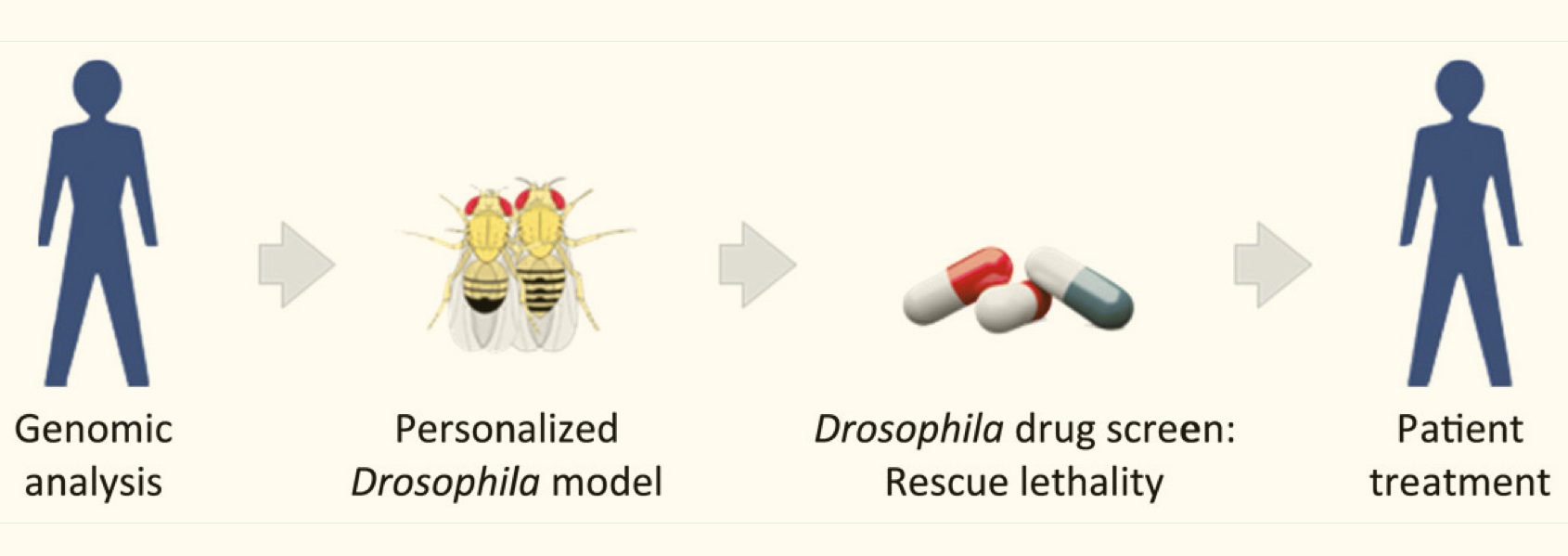
/The promise of personalized medicine has been limited by at least two factors: 1) the power of our models—including our ability to get and process data, and 2) our ability to test potential therapeutic solutions in meaningful biological systems, rapidly and systematically, before testing in humans. While we’re seeing rapid and predictable improvements with the power of our models corresponding improvements in how we test therapies have been less consistent. Even if you have a good, genome-level understanding of an individual’s cancer, there is no guidebook for what treatment will be effective against that particular type of cancer. One promising approach has been to use fruit flies (Drosophila) genetically-modified with similar mutational loads as cancer patients to test the efficacy of drug combinations for suppressing tumor growth.
Ross Cagan is an exceptionally creative researcher who leads a lab at the Icahn School of Medicine at Mount Sinai and has been using fruit flies to develop personalized medicine approaches for over 10 years. This week, the Cagan lab published one of the first examples of clinically effective therapies based on such an approach, with lead author Dr. Erdem Bangi (see below for abstract and link). This article describes how Drosophila were screened for effective drug combinations for inhibiting tumors with a similar composition and complexity as a terminally ill colorectal cancer patient’s tumors, and how a specific drug combination was identified and used to effectively shrink the patient’s tumors. Though this treatment did not provide a permanent cure, it did appear to extend the patient’s life and is important proof that this type of approach can be effective.
Excellent, more in-depth summaries can be found HERE and HERE and HERE, so check them out.
The original article, with its somewhat difficult to penetrate title…
Bangi, E., et al. (2019) A personalized platform identifies trametinib plus zoledronate for a patient with KRAS-mutant metastatic colorectal cancer. Science Advances
Abstract
Colorectal cancer remains a leading source of cancer mortality worldwide. Initial response is often followed by emergent resistance that is poorly responsive to targeted therapies, reflecting currently undruggable cancer drivers such as KRAS and overall genomic complexity. Here, we report a novel approach to developing a personalized therapy for a patient with treatment-resistant metastatic KRAS-mutant colorectal cancer. An extensive genomic analysis of the tumor’s genomic landscape identified nine key drivers. A transgenic model that altered orthologs of these nine genes in the Drosophila hindgut was developed; a robotics-based screen using this platform identified trametinib plus zoledronate as a candidate treatment combination. Treating the patient led to a significant response: Target and nontarget lesions displayed a strong partial response and remained stable for 11 months. By addressing a disease’s genomic complexity, this personalized approach may provide an alternative treatment option for recalcitrant disease such as KRAS-mutant colorectal cancer.
Thank you for reading!